Residue-Based Thermogravimetric Analysis: A Novel Method to Quantify Carboxylate Group Modifications in Macromolecules
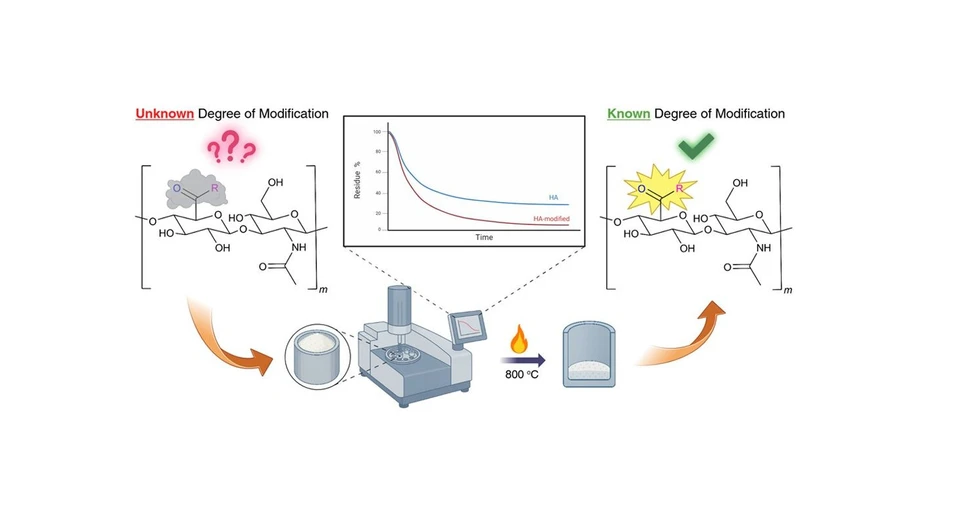
Biomacromolecules 2025, 26, 11, 7767–7777: Graphical abstract
Quantifying the degree of modification (DoM) of hyaluronic acid (HA) is a key step in biomaterials research, yet diverse functional groups often limit the precision of spectroscopic approaches. This study presents a residue-based thermogravimetric analysis (TGA) method that determines DoM by comparing the thermal decomposition residues of sodium hyaluronate (NaHA) and carboxylate-modified HA derivatives. The inorganic residue (Na₂CO₃) obtained as the final product serves as a reliable indicator of modification, independent of the attached substituent.
The method was validated on four HA derivatives—aldehyde, furan, thiol, and cyanoacetate. Three derivatives showed excellent agreement with ^1H NMR and UV–vis data, while the cyanoacetate sample, previously unquantifiable by conventional methods, was characterized successfully for the first time. Because the residue originates solely from Na⁺, this TGA approach is broadly applicable to any carboxylate-bearing polymer. Residue-based TGA therefore fills an important analytical gap, offering a label-free, structure-independent tool capable of quantifying even “silent” functional groups relevant in biomaterials development.
The original article
Residue-Based Thermogravimetric Analysis: A Novel Method to Quantify Carboxylate Group Modifications in Macromolecules
Christos Leliopoulos, Hamidreza Mokhtari, Shima Tavakoli, Oommen P. Varghese*
Biomacromolecules 2025, 26, 11, 7767–7777
https://doi.org/10.1021/acs.biomac.5c01271
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Biomaterials derived from extracellular matrix (ECM)-based sources have been extensively explored to develop injectable fillers for esthetic treatments, as well as to fabricate advanced hydrogels for tissue engineering, regenerative medicine, and sophisticated drug-delivery systems. (1−3) This broad range of applications highlights the urgent need for accurate, reliable, and straightforward methods to track chemical modifications in biomaterials, particularly driven by stringent regulatory requirements in medical device development, where precise documentation and rigorous control of material alterations are critical. (4,5)
TGA, an established thermal-analysis technique widely used in polymer laboratories, precisely measures mass changes in materials subjected to controlled temperature or atmospheric conditions, providing valuable insights into thermal stability, decomposition kinetics, and compositional details. TGA has been extensively utilized for characterizing polymer blends, evaluating thermal degradation profiles, quantifying residual solvents or moisture critical for storage and processing, investigating additive effects (e.g., flame retardants and stabilizers), and assessing polymer purity. In polymer research specifically, TGA has proven to be crucial in elucidating dehydration and decomposition mechanisms and evaluating the impact of chemical modifications on thermal stability.
Building upon these precedents, we propose a novel TGA-based methodology to quantify chemical modifications in NaHA by measuring residue loss. In our approach, NaHA is completely oxidized at high temperatures, leaving behind a predictable residue (Na2CO3) that is directly proportional to the remaining free carboxylate groups. Thus, this residue measurement provides a straightforward and accurate quantification inversely related to the extent of modification. Our investigation covered four chemically modified HA derivatives: aldehyde, furan, thiol, and cyanoacetate groups, encompassing spectroscopically “visible” and spectroscopically “silent” scenarios. By rigorously comparing TGA-derived quantitative results against established NMR and UV-based analyses and employing unmodified HA as a control, we validate the accuracy, reliability, and broader applicability of TGA residue analysis for the precise determination of DoM. The side-by-side evaluation of spectroscopically “visible” and “silent” modifications further demonstrates TGA as an agnostic tool to determine the DoM where traditional methods such as FTIR, NMR, or UV–vis studies fail. We believe that our strategy has general applicability and could be applied to different types of macromolecules and even smaller molecules, potentially even those that do not have a well-defined structure.
2. Experimental Section
2.4. Procedure for TGA Analysis
Thermal analysis was conducted using a TGA/DSC 3+ instrument (Mettler Toledo AB, Stockholm, Sweden) equipped with an autosampler, an SDTA sensor, a large furnace, and an XP5U balance, suitable for crucibles and samples up to 900 μL and 5 g, respectively. Samples of ≈20 mg (range: 15–25 mg) were placed in alumina crucibles (300 μL capacity) fitted with lids to prevent sample loss. The 300 μL crucible was chosen so that the sample was not densely packed, thereby avoiding foaming from the decomposition gases. While an increased sample mass enhances the signal-to-noise ratio, it can also induce thermal gradients within the 300 μL pan. The influence of such thermal gradients is effectively compensated for by employing extended durations for the experimental segments. The instrument was calibrated using TGA-specific calibration weights (CarePac, class E2, Mettler Toledo). Drift and noise tests verified the instrument’s performance according to the manufacturer’s specifications. A sample weight at or above the USP-recommended minimum of 1.7 mg was targeted to minimize measurement error.
2.5. Characterization of TGA Residues
Residues from TGA were characterized by using FTIR and scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX).
For FTIR analysis, spectra were recorded with an IRTracer-100 spectrometer (Shimadzu), scanning from 400 to 4000 cm–1 at a resolution of 4 cm–1, with 45 scans per sample. Residues were analyzed as powders obtained by scraping crucibles. Commercially available Na2CO3 served as a reference to verify the residue composition, while commercially available NaCl and NaOH were used as controls.
For SEM-EDX analysis, the presence of Na+ within the residues and their corresponding micromorphology were investigated by Scanning Electron Microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX). A Zeiss Merlin Field Emission Gun Scanning Electron Microscope (FEG-SEM), operated at an accelerating voltage of 10 kV, was utilized for these analyses. Samples were analyzed using standard operating protocols for SEM imaging and elemental composition analysis. The samples for SEM and EDX analysis were prepared by placing residue particles onto double-sided conductive carbon tape. To prevent charging effects during imaging, a thin gold coating (∼5 nm) was sputtered onto the sample surface prior to analysis. The surfaces and cross-sections of the samples were examined using a field emission scanning electron microscope (FEG-SEM, Zeiss Merlin). For imaging, secondary electron (SE) detectors, specifically in-lens annular-type detectors, were employed to capture high-resolution surface morphology. Elemental analysis and X-ray mapping were conducted using the same SEM equipped with an X-Max 80 mm2 Silicon Drift EDX Detector (Oxford Instruments), which has high sensitivity for analysis at elevated count rates. Data acquisition and analysis were performed using Oxford AZtec software. Elemental mapping was performed to assess the distribution of elements across the sample and quantification was achieved through point analysis and spectral fitting.
3. Results and Discussion
3.1. Synthesis of HA Derivatives
To evaluate a residue-based approach for quantifying carboxylate substitution, we synthesized various chemically modified NaHA, namely HA derivatives having aldehyde-, furan-, thiol-, and cyanoacetate modifications, denoted as HA-Ald, HA-Furan, HA-Thiol, and HA-Cyano, respectively. We employed carbodiimide chemistry to conjugate different functional groups, specifically at pH 4.7 for hydrazide-modified reagents (23) and pH 6 for amine-modified reagents (Scheme 1). (20) Characterization of these derivatives was carried out using conventional techniques as described earlier. HA-Furan and HA-Thiol each displayed a unique NMR resonance that allows the classical determination of the DoM, whereas HA-Ald could be quantified only after an additional derivatization reaction. The DoM of HA-Thiol was also measured with Ellman’s assay using UV–vis. However, as discussed above, HA-Cyano lacks any validated analytical protocol, representing a spectroscopically “silent” modification.
 Biomacromolecules 2025, 26, 11, 7767–7777: Scheme 1. Schematic Representation of NaHA and Its Chemical Modifications. The native NaHA disaccharide unit contains sodium carboxylate groups that serve as reactive sites for derivatization. HA derivatives were synthesized using coupling carbodiimide chemistry to obtain aldehyde, furan, thiol, and cyanoacetate groups that represent spectroscopically visible and silent modifications, as shown.
Biomacromolecules 2025, 26, 11, 7767–7777: Scheme 1. Schematic Representation of NaHA and Its Chemical Modifications. The native NaHA disaccharide unit contains sodium carboxylate groups that serve as reactive sites for derivatization. HA derivatives were synthesized using coupling carbodiimide chemistry to obtain aldehyde, furan, thiol, and cyanoacetate groups that represent spectroscopically visible and silent modifications, as shown.
3.2. TGA as a Method to Determine DoM of HA Derivatives
To accurately quantify the extent of chemical modification in HA, we leveraged a fundamental property inherent to carboxylic acids’ tendency to form carboxylate ions under elevated pH conditions. These negatively charged carboxylate ions readily establish ionic interactions, known as salt bridges, with various cations. Commercially, Na+ is the predominantly used counterions for HA formulations, although K+, Ca2+, and Mg2+ can also be used, as they can form stable electrostatic associations with the carboxylate groups.
The intrinsic acidity of HA, characterized by a pKa of approximately 3.2, (26) underscores the relevance of precise pH control. The extent of dissociation can be estimated from the Henderson–Hasselbalch relationship (eqs S1–S3).
As illustrated in Figure 1A and Table S1, at pH 3.2, HA exhibits 50% ionization, whereas at physiological and higher pH levels (≥7.2), ionization dramatically increases to over 99.99%.
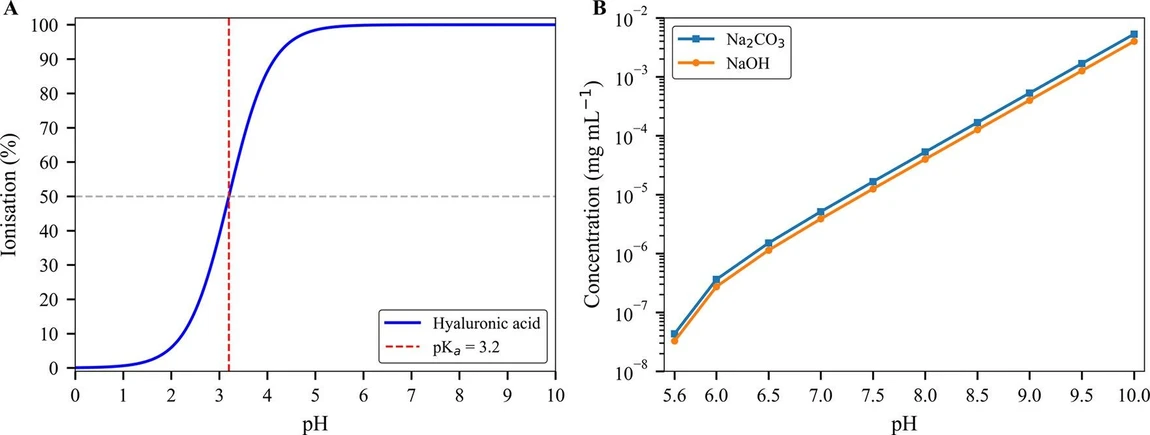 Biomacromolecules 2025, 26, 11, 7767–7777: Figure 1. Ionization behavior, buffer capacity, and TGA methodological framework. (A) Theoretical ionization profile of the carboxyl functional group in HA as a function of pH, modeled using the Henderson–Hasselbalch equation with a pKa of 3.2. This curve illustrates the extent of ionization (% deprotonation) across physiologically relevant pH values. (B) Log-scale plot of the NaOH concentration required to raise the pH from an initial value of 5.6 (typical of DI water) to progressively higher values. For comparison, the theoretical concentration of Na2CO3 is also plotted, assuming complete conversion of all Na+ to Na2CO3.
Biomacromolecules 2025, 26, 11, 7767–7777: Figure 1. Ionization behavior, buffer capacity, and TGA methodological framework. (A) Theoretical ionization profile of the carboxyl functional group in HA as a function of pH, modeled using the Henderson–Hasselbalch equation with a pKa of 3.2. This curve illustrates the extent of ionization (% deprotonation) across physiologically relevant pH values. (B) Log-scale plot of the NaOH concentration required to raise the pH from an initial value of 5.6 (typical of DI water) to progressively higher values. For comparison, the theoretical concentration of Na2CO3 is also plotted, assuming complete conversion of all Na+ to Na2CO3.
3.4. Calibration of Native HA to Optimize TGA Method for Quantification
One of the challenges of using TGA as a method for quantification includes inherent organic or inorganic impurities that can affect quantification. We therefore decided to first determine the experimental carboxylate residue and compare it with anticipated values from a theoretically 100% HA. Of note, most commercially available NaHA has 5–10% impurities that need to be calibrated to remove systematic measurement errors. For the quantification, we adjusted the pH of a small-volume solution to obtain a target pH range of 7.5 to 8.0 for the HA solution, ensuring a carboxylic acid ionization degree above 99.995% and minimizing the concentration of free Na+. To evaluate reproducibility across different sample sizes, we analyzed varying masses (15 mg, 20 mg, and 25 mg) of unmodified NaHA. As illustrated in Figure 3, the measurements showed excellent correlation, prompting us to calculate a mean residue from these nine measurements, resulting in an average residue of 12.56% for the unmodified NaHA. The residue of different sodium-containing organic molecules has been consistently identified as Na2CO3 in existing literature. (28)
 Biomacromolecules 2025, 26, 11, 7767–7777: Figure 3. TGA of unmodified pH-adjusted NaHA representing percentage mass loss curves for three different initial sample masses (15, 20, and 25 mg), n = 3 for each, showing excellent reproducibility. The final residue plateau is enlarged in the inset to highlight the minimal variance between samples and confirm consistent TGA performance.
Biomacromolecules 2025, 26, 11, 7767–7777: Figure 3. TGA of unmodified pH-adjusted NaHA representing percentage mass loss curves for three different initial sample masses (15, 20, and 25 mg), n = 3 for each, showing excellent reproducibility. The final residue plateau is enlarged in the inset to highlight the minimal variance between samples and confirm consistent TGA performance.
To experimentally confirm the identity of the residue, we performed a series of analytical techniques (Figure 4). To confirm the residue, we first performed scanning electron microscopy (SEM) coupled with SEM-EDX. SEM images of the TGA residue (Figure 4A), elemental mapping (Figure 4B), and quantitative elemental analysis (Figure 4C) collectively revealed a Na+ content of approximately 99.2%. It should be noted that routine EDX cannot reliably quantify carbon or oxygen, and therefore the expected CO32– partners are not represented in this measurement. Additionally, we conducted FTIR analyses, as displayed in Figure 4D, and compared the residue with pure Na2CO3 powder. Thus, this validates the residue as nearly pure Na2CO3. The presence of carbon, aluminum, and gold in the EDX spectra (Figure 4C) is from the carbon tape, the aluminum sample holder/stage, and the gold coating, respectively.
 Biomacromolecules 2025, 26, 11, 7767–7777: Figure 4. Characterization of Na2CO3 in the TGA residue. (A) SEM image showing the surface morphology of the sample after thermal degradation, with visible inorganic crystalline structures. (B) EDX elemental mapping highlighting the distribution of Na+ (cyan), confirming the widespread presence of Na2CO3. (C) EDX spectrum of the residue showing major elemental peaks for Na+, consistent with the composition of Na2CO3. (D) FTIR spectra comparing commercial Na2CO3 with the TGA residue, showing matching characteristic peaks and confirming the identity of the residue as Na2CO3.
Biomacromolecules 2025, 26, 11, 7767–7777: Figure 4. Characterization of Na2CO3 in the TGA residue. (A) SEM image showing the surface morphology of the sample after thermal degradation, with visible inorganic crystalline structures. (B) EDX elemental mapping highlighting the distribution of Na+ (cyan), confirming the widespread presence of Na2CO3. (C) EDX spectrum of the residue showing major elemental peaks for Na+, consistent with the composition of Na2CO3. (D) FTIR spectra comparing commercial Na2CO3 with the TGA residue, showing matching characteristic peaks and confirming the identity of the residue as Na2CO3.
The FT-IR comparison (Figure 4D) shows that, across the 4000–500 cm–1 region, the spectrum of the TGA residue (orange) is virtually superimposable on the sodium carbonate reference (blue). Such complete overlap is the qualitative hallmark indicating that both traces correspond to the same carbonate compound.
Elemental analysis by SEM-EDX further corroborates that the residue is a sodium-rich carbonate phase. The elemental map (Figure 4B) is dominated by Na+ (rendered cyan), and the quantitative spectrum (Figure 4C) attributes ≈99 wt % of the detected signal to Na+, with only trace K+ and no detectable Ca2+ or Mg2+. Because EDX cannot quantify carbon or oxygen reliably, the expected CO32– ions are invisible. Nevertheless, the absence of Ca2+ rules out CaCO3, and the lack of Cl– eliminates NaCl. We therefore believe that Na2CO3 is the only composition consistent with the observed elemental distribution, allowing us to quantitatively determine the DoM.
4. Conclusion
This work presents a residue-based TGA protocol that quantifies chemical modification in carboxylate-bearing polysaccharides simply by weighing what remains after complete thermal oxidation. By converting HA to its sodium salt, correlating the residual Na2CO3 mass with unreacted carboxylate groups, and performing milligram-scale TGA runs followed by blank subtraction, the method transforms the long-standing challenge of “measuring what is added” into the easier and modification-agnostic task of “measuring what is lost.”
When applied to four structurally diverse HA derivatives─namely aldehyde, furan, thiol, and the “spectroscopically silent” cyanoacetate─the protocol yielded mean DoM values that deviated by <1% from the values obtained by 1H NMR, while maintaining an instrumental uncertainty of ≤0.06%. Because the workflow needs only one pH-adjustment step and minimal sample, single-run measurements are justified when material is scarce and sample preparation is not easy.
Although demonstrated with HA, the approach can be inherently transferred to any carboxylate-containing polymer formulated as a Na+ salt. Its simplicity, label-free operation, and subpercent precision address key regulatory concerns of lot-to-lot consistency, method traceability, and suitability for gels or solids, without relying on complementary spectroscopic assays. Residue-based TGA thus offers a robust, universally accessible tool for the quantitative characterization of chemically modified polysaccharides in both biomedical and industrial settings, closing a critical analytical gap and accelerating product development and standardization.