Microwave-Assisted Digestion Method and Dispersive Magnetic Solid-Phase Microextraction for the Determination of Major and Trace Elements in Lignocellulosic Biomass by ICP-OES
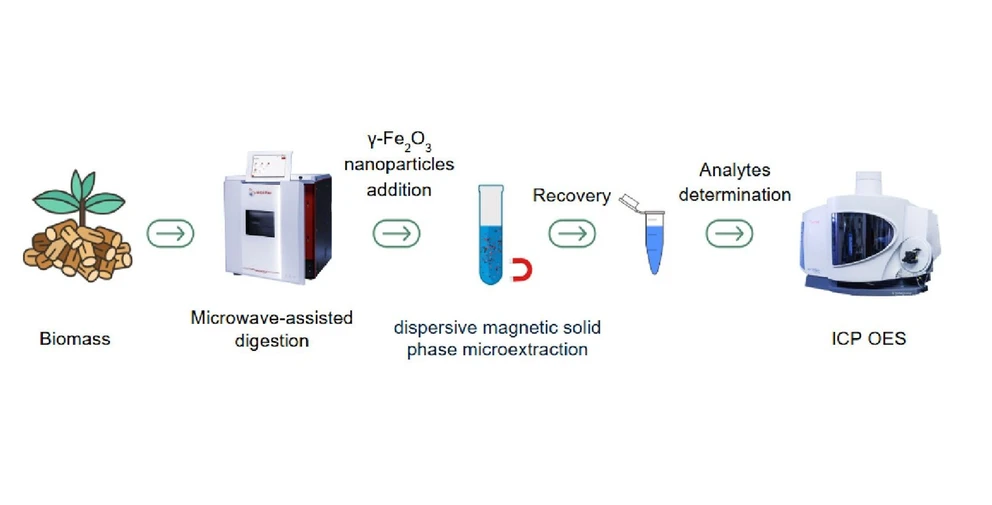
ACS Omega 2025, 10, 28, 30443–30449: Graphical abstract
This study presents a combined microwave-assisted digestion (MAD) and dispersive magnetic solid-phase microextraction (DMSPE) method for determining major (Ca, Cu, Fe, Mn, Mg, Na, Zn) and trace elements (As, Pb, Se) in lignocellulosic biomass by ICP-OES. Maghemite nanoparticles were synthesized and characterized to serve as sorbents, and conditions for both MAD and DMSPE were optimized using a central composite design.
The method achieved low detection limits (As 0.01, Pb 0.03, Se 0.01 µg g–1) and high enrichment factors (21–42 for trace elements). Validation with certified reference materials confirmed accuracy. Application to five biomass samples provided reliable quantification of major elements, while As, Pb, and Se were below LOQs. This approach offers a robust strategy for elemental profiling of lignocellulosic biomass.
The original article
Microwave-Assisted Digestion Method and Dispersive Magnetic Solid-Phase Microextraction for the Determination of Major and Trace Elements in Lignocellulosic Biomass by ICP-OES
Camilla M. Belmiro, Mikaelle de Carvalho Gomes, Fernanda Nunes Ferreira, Márcia Angelica F. S. Neves, and Jefferson Santos de Gois*
ACS Omega 2025, 10, 28, 30443–30449
https://doi.org/10.1021/acsomega.5c02196
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Lignocellulosic biomass consists mainly of cellulose, hemicellulose, and lignin. It can be used as an ecological material for the production of second-generation fuels, such as ethanol from petroleum to replace fossil fuels and their derivatives, and as an energy source (by gasification, pyrolysis, liquefaction, or combustion), with the aim of contributing to the reduction of global CO2 emissions. (1−4)
A variety of analytical techniques can be used for the elemental characterization of biomass and biomass-derived ash, such as inductively coupled plasma mass spectrometry (ICP–MS), (5,6) flame atomic absorption spectrometry (FAAS), (7) graphite furnace atomic absorption spectrometry (GF AAS), (3) laser-induced breakdown spectroscopy (LIBS), (8) and inductively coupled plasma optical emission spectrometry (ICP-OES). (9−11)
Inductively coupled plasma optical emission is often used for the determination of trace elements due to its low detection limits and multielement capability. However, this technique usually requires at least one sample preparation step to solubilize the analytes in an aqueous medium prior to analysis. Microwave-assisted acid digestion (MAD) in closed vessels is considered the most efficient technique for the decomposition/solubilization of organic sample matrices and the determination of trace elements by spectrometric techniques. (12) Concentrated acids and their mixtures have been identified for the preparation of biomass samples with MAD, the most commonly used acids are hydrofluoric acid, (10) nitric acid, and/or hypochlorous acid and their mixtures; (13) hydrogen peroxide may also be used to recycle oxygen into the aqueous media. (4,6)
Elements such as As, Pb, and Se usually occur in very low concentrations (ng g–1) and therefore require very sensitive analytical techniques for their determination or preconcentration methods, which can provide a cost-effective method for their determination. Dispersive magnetic solid-phase extraction (DMSPE) with magnetic nanoparticles of iron oxide (Fe3O4 and Fe2O3) is advantageous because they can be quickly obtained by synthesis, can adsorb elements, and can be easily separated from the aqueous medium with a magnet. (14,15)
Therefore, the aim of this work was to develop a MAD method using diluted HNO3 for the determination of trace elements (Ca, Cu, Fe, Mn, Mg, Na, Zn), followed by an MSPE preconcentration method for the determination of As, Pb, and Se in lignocellulosic biomass samples by ICP-OES.
2. Materials and Methods
2.1. Instrumentation
An ICP-OES model iCAP 6300, equipped with a Mira Mist nebulizer (Burgener Research Inc., Canada) and a cyclone spray chamber (Thermo Scientific, USA), was used for the multielement determination. The operating parameters used in ICP-OES were plasma gas flow (12 L min–1), nebulizer gas flow (0.4 L min–1), auxiliary gas flow (1.0 L min–1), radio frequency power (1300 W), pump flow rate (5 rpm, 0.2 mL min–1), and radial view. The monitored wavelengths were Ca (422.673 nm), Cu (324.754 nm), Fe (259.940 nm), Mn (257.610 nm), Mg (280.270 nm), Na (588.995 nm), Zn (213.856 nm), As (189.042 nm), Pb (220.353 nm), Se (203.985 nm), and Sc (361.384 nm) as internal standards. Argon with a purity of 99.95% (Air Liquide, Brazil) was used as the main, auxiliary, and nebulizer gas. All mass measurements were performed using an analytical balance with an accuracy of 0.1 mg, model M214A (Bel Engineering, Italy). The samples were ground in a mill model MG200 (Black+Decker, Brazil) and sieved with a 200 mesh sieve.
The microwave-assisted digestion of the samples was carried out in a Multiwave PRO microwave oven (Anton-Paar, Graz) with a 24HVT50 rotor model. The DMSPE experiments were carried out in a horizontal orbital shaker model SK-180-PRO (Scilogex, EUA) and a thermal shaker model Thermo Mixer (Kasvi, Brazil).
2.5. Dispersive Magnetic Solid-Phase Microextraction Procedure
2.5.2. Characterization of the γ-Fe2O3 Magnetic Nanoparticles
The synthesized magnetic γ-Fe2O3 nanoparticles were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (FEG-SEM), and thermogravimetric analysis (TGA). A Frontier FTIR of the 98737 series (PerkinElmer, USA) was used for the FTIR analysis. The analysis was performed with a resolution of 4 cm–1 in the range of 4000 to 400 cm–1 in KBr pellets. For TGA, an SDT Q600 model (TA Instruments, USA) was used with the following parameters: a temperature range from 30 to 700 °C at a rate of 20 °C min–1 under a nitrogen atmosphere (150 mL min–1). For FEG-SEM, images were taken with an electronic microscope model JSM-7100F (Jeol, Japan) with magnifications of x30 000 and x60,000. The XRD was carried out in an AXS D-5005/Siemens (Bruker, EUA), with an angular step of 0.10°, ranging from 15 to 70°, and with an acquisition time between counts of 1.0 s.
3. Results and Discussion
3.1. Chemical Characterization of the γ-Fe2O3 Magnetic Nanoparticles
The X-ray diffractogram of the magnetic γ-Fe2O3 nanoparticles is shown in Figure 1A. Diffraction peaks were consistent with the standard structure and indicated the presence of the cubic phase of maghemite. These reflections are assigned to (220), (311), (400), (422), (511), and (440), which correspond to the crystallographic planes that are characteristic reflections of maghemite, indicating that they are consistent with the X-ray diffraction pattern of γ-Fe2O3. (17)
 ACS Omega 2025, 10, 28, 30443–30449: Figure 1. X-ray diffraction (A), Fourier transform infrared spectra (B), scanning electron microscopy x60,000 (C), and thermogravimetric analysis of γ-Fe2O3 (D).
ACS Omega 2025, 10, 28, 30443–30449: Figure 1. X-ray diffraction (A), Fourier transform infrared spectra (B), scanning electron microscopy x60,000 (C), and thermogravimetric analysis of γ-Fe2O3 (D).
Fourier transform infrared spectroscopy was used to characterize the functional groups. The FTIR spectra of the γ-Fe2O3 nanoparticles, shown in Figure 1B, highlight the bands at 628, 580, and 447 cm–1, which correspond to the vibrational mode of stretching and angular deformation characteristic of Fe–O bonding and confirm the formation of γ-Fe2O3. (18) The broad stretching band at 3411 cm–1 corresponds to the OH group and surface H2O, and the band at 1630 cm–1 correlates with the angular deformation mode of H–O–H. The OH groups at the surface are associated with residual or physically adsorbed water. (14)
The thermal stability of maghemite was investigated using TGA. It was found that the material is thermally stable, as it suffers a slight mass loss with an increase in temperature, as shown in Figure 1D. The mass loss at around 100 °C is due to the loss of water, which is physically adsorbed onto the material. (16) In the range between 550 and 580 °C, a mass loss is observed, which is due to phase transitions from maghemite to hematite. (15)
3.2. Microwave-Assisted Digestion of Biomass
Microwave-assisted digestion is a powerful technique for decomposition of the organic matrix of a sample and solubilization of inorganic analytes into the aqueous media for analysis. This technique can be used with inorganic acids and H2O2 to increase the efficiency of sample digestion. Microwave-assisted digestion in closed vessels can minimize analyte loss, reduce contamination, and shorten sample preparation time. It also offers high sample decomposition efficiency and high sample throughput. (19,20) The selection of the type and concentration of inorganic acids, as well as the sample mass, is crucial for sample preparation in microwave-assisted digestion. Therefore, the optimization of these factors was performed, as described in Section 2.3. Nitric acid was selected for sample preparation due to its oxidizing properties and versatility for a variety of organic matrices; furthermore, the nitrates are soluble in aqueous media.
Table S3 shows the fitted models for each analyte after refinement, where the obtained R-squared ranged from 0.6885 to 0.9989, while the fitted R-squared ranged from 0.6719 to 0.9987, indicating a good fit of the models. According to the Shapiro–Wilk test (α = 0.05), the residuals of the models for Ca, Fe, Mg, Mn, Na, and Zn were found to follow a normal distribution, except for Cu, for which the central limit theorem was considered.
The desirability function was applied to determine the optimal experimental condition for multiresponse optimization, with values ranging from 0 (undesirable value) to 1 (desirable value). (21) The surface response of the overall desirability for the microwave-assisted digestion method is shown in Figure 2. The optimum experimental condition was achieved at a sample mass of 280 mg, 2.5 mL of HNO3, and 2.0 mL of H2O2, achieving an overall desirability of 0.534, which is an acceptable value, while the individual desirabilities were Ca (0.618), Cu (0.535), Fe (0.428), K (0.388), Mg (0.378), Mn (0.866), Na (0.620), and Zn (0.591).
 ACS Omega 2025, 10, 28, 30443–30449: Figure 2. Overall desirability response surface for the optimization of the microwave-assisted digestion of lignocellulosic biomass and determination of Ca, Cu, Fe, Mn, Mg, Na, and Zn by ICP-OES.
ACS Omega 2025, 10, 28, 30443–30449: Figure 2. Overall desirability response surface for the optimization of the microwave-assisted digestion of lignocellulosic biomass and determination of Ca, Cu, Fe, Mn, Mg, Na, and Zn by ICP-OES.
4. Conclusions
A microwave-assisted digestion method and a dispersive magnetic solid-phase microextraction method for trace-element determination in lignocellulosic biomass were successfully obtained. Both methods were optimized using a multivariate approach, which ensured a high efficiency of the methods.
The developed microwave-assisted digestion achieved good accuracy using a lower concentration of reagents than reported in the literature, while the microextraction method using maghemite nanoparticles provided a simple and reliable alternative for the determination of trace elements in biomass samples. Although only Ca, Cu, Fe, Mn, Mg, Na, Zn, As, Pb, and Se were considered in the validation of the method, we believe that this method can be extended for other analytes after proper validation, especially As, Pb, and Se, since the recovery of the analytes was performed by dissolving the nanomaterial into an aqueous medium. The methods provided good accuracy for all analytes and LOQs suitable for the purpose and proved to be a good alternative for the determination of trace elements in biomass samples from lignocellulose.