Influence of Chemical Profile on the Antioxidant Capacity of Brazilian Stingless Bee Honey
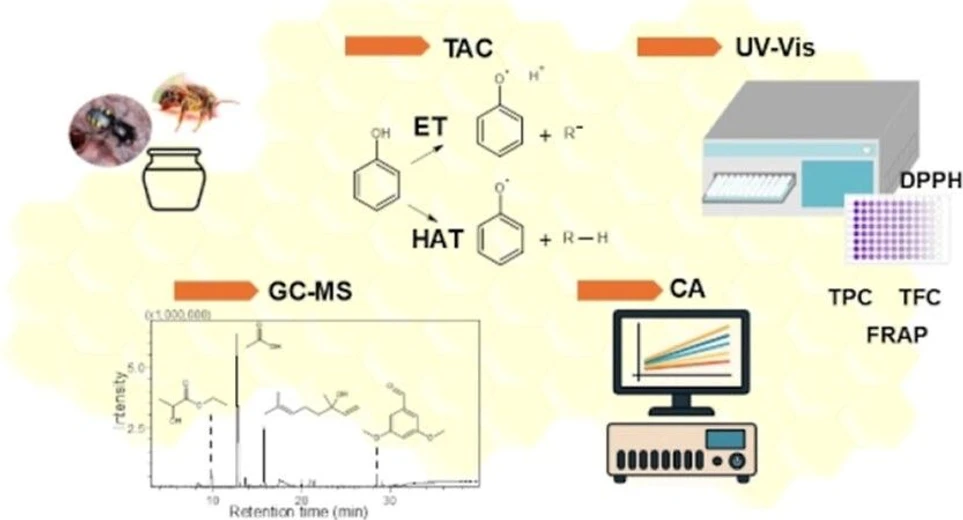
ACS Omega 2025, 10, 20, 20550–20561: Graphical abstract
This study explored the chemical composition and antioxidant capacity of stingless bee honey (SBH) produced by Melipona species in Espírito Santo, Brazil. Using FT-MIR and NMR spectroscopy combined with chemometrics, clear separation between M. capixaba and M. quadrifasciata was observed.
Volatile compounds identified by GC–MS included carboxylic acids, ketones, aldehydes, and alcohols, with simple phenols such as p-cresol and allyl guaiacol also detected. Phenolic and flavonoid contents correlated selectively with antioxidant capacity, especially between total phenolics and CRAC. These findings highlight chemometrics combined with phenolic profiling as a robust approach for quality control and standardization of SBH.
The original article
Influence of Chemical Profile on the Antioxidant Capacity of Brazilian Stingless Bee Honey
Lucas R. de O. Dias, Bruna M. Damm, Bruno F. Paqueli, Bruno Q. Araújo, Gislane C. Oliveira, Diolina M. Silva, Eustaquio V. R. de Castro, Rafael de Q. Ferreira*, and Álvaro C. Neto
ACS Omega 2025, 10, 20, 20550–20561
https://doi.org/10.1021/acsomega.5c01134
licensed under CC-BY 4.0
Selected sections from the article follow. Formats and hyperlinks were adapted from the original.
Stingless bees (Meliponini), also known as native bees, are found in tropical and subtropical regions representing more than 500 bee species worldwide. (1) Although international legislation governs the quality and identity parameters of Apis melliferahoney, there is a need to establish specific standards and regulatory guidelines for stingless bee honey (SBH). (2) Currently, SBH quality is assessed based on its physicochemical, sensory, and microbiological properties. (3)
In general, honey is a complex mixture of sugars (primarily glucose and fructose), and its chemical composition can vary considerably depending on climatic conditions, stages of maturation, bee species, and flower type as well as processing and storage methods. (4)
SBH is recognized for its characteristic sweetness combined with an acidic taste, a more fluid texture, and slow crystallization. In addition to sugars, enzymes, amino acids, organic acids, minerals, volatile organic compounds, pigments, and pollen grains, SBH contains phenolic compounds that can impact the antioxidant capacity. (3) Due to the antioxidant, nutritional, and biological properties of SBH, it has attracted economic interest in the past few years. (5,6)
Phenolic compounds are widely distributed in plants from bee pastures. (7) Several phenolic acids and flavonoids, such as gallic acid, vanillic acid, protocatechuic acid, cinnamic acid derivatives, quercetin, and rutin have been identified in SBH. These molecules contribute to honey’s flavor, aroma, and antioxidant capacity. (8)
Furthermore, several stingless bee species are capable of producing phenolic compounds-rich honey. Therefore, the chemical characterization of this natural product is essential to know its compositional and functional profiles. (9)
In 2020, Kozłowicz and collaborators characterized honey derived from chestnut flowers and honey with dark orange berries, both produced by stingless bees, identifying phenolic compounds using gas chromatography–mass spectrometry (GC–MS) and Fourier transform mid-infrared (FT-MIR) spectroscopy. Several chemical compounds were identified, including 3-phenylpropionic acid and ferulic acid in both samples, while m-coumaric acid, benzoic acid, and cinnamic acid were predominantly found in only one honey sample. (10)
The presence of phenolic compounds in honey contributes to its antioxidant responses. In general, the antioxidant capacity can be determined by using spectrophotometric methods such as ferric reducing antioxidant power (FRAP) and DPPH (2,2-diphenyl-1-picrylhydrazyl). (11,12) Some works have determined the total phenolic and flavonoid contents and verified their correlations. While spectrometric methods are widely employed for the quantification of phenolic compounds and the evaluation of antioxidant capacity, the accuracy of these assessments can be compromised by matrix effects and interfering substances, resulting in weak correlations between measured properties. (13)
Consequently, alternative approaches are necessary to verify the correlation among these physicochemical characteristics. Ceric reducing antioxidant capacity (CRAC) electrochemical assay offers several advantages over spectrophotometric techniques. These include the ability to specifically target the redox state of antioxidants, low cost, potential for miniaturization, rapid analysis when compared to reference methods, insensitivity to light, and the absence of interference from sample coloration. (14)
The objective of the present study is to conduct detailed chemical characterization combined with an evaluation of antioxidant capacity to ensure authenticity and quality control of honey. This knowledge is crucial to addressing existing gaps, encouraging proper regulation, and promoting sustainability in the production of SBH. Consequently, it enables broader acceptance and distribution of this product, benefiting consumers, producers, and biodiversity.
2. Materials and Methods
2.3. Fourier Transform Mid-infrared (FT-MIR) Analyses
For physicochemical characterization using FT-MIR, a drop of SBH was directly applied in the attenuated total reflectance (ATR) module (Cary 630, Agilent Technologies Inc., USA). FT-MIR spectra were acquired from 4000 to 650 cm–1 with 32 scans and a resolution of 4 cm. ATR module was cleaned with analytical-grade isopropyl alcohol for each sample. (16)
2.4. Nuclear Magnetic Resonance (NMR) Analyses
SBH samples (20.0 mg) were dissolved in D2O. 1H NMR spectra were obtained on a 400 MHz spectrometer (VNMRS 400 model, Varian, USA) operating at a magnetic field of 9.4 T, using a probe of 5 mm 1H/X/D Broadband. All spectra were processed using MestReNova software. (17)
2.5. Identification of Volatile Organic Compounds by Static Headspace Sampling and Gas Chromatography–Mass Spectrometry (SHS-GC-MS)
The volatile fraction of SBH was analyzed using a GC-MS QP2010 Ultra system (Shimadzu Corporation, Japan) equipped with a static headspace sampler (SHS) and an OV-WAX capillary column (30 m length, 0.25 mm internal diameter, and 0.25 μm film thickness). A 20 mL headspace vial containing 2 g of the sample was incubated in an SHS oven at 95 °C for 15 min. Subsequently, 1 mL of the volatile fraction was collected using a 2.5 mL syringe (Hamilton, USA) and injected in splitless mode (0.45 min) at an injector temperature of 240 °C. The oven temperature program had an initial temperature of 40 °C for 2 min, ramp of 4 °C min–1 to 120 °C, and a heating rate of 11 °C min–1 to 230 °C, where it was held for 8 min. Helium (99.999%, White Martins, Brazil) was used as the carrier gas at a flow rate of 1.5 mL min–1. The mass spectrometer operated in scan mode over a mass range of 35–300 Da, with an ion source temperature of 230 °C, an interface temperature of 220 °C, and a solvent cutoff time of 2.5 min. Chromatographic data were processed using LabSolutions GC–MS Solutions software, version 4.20 (Shimadzu Corporation, Japan). Volatile compound identification was performed by comparing the experimental mass spectra with those in the NIST11 and WILEY7 libraries. Results were expressed as relative area (%) by the integration of peaks in the total ion chromatogram (TIC). (18) Retention index (RI) was calculated using the equation RI = 100 (n) + 100 (m – n) (tRi – tRn)/(tRm – tRn), where m and n are the numbers of carbons of the n-alkanes that elute before and after “i”, respectively, and “i” is a VOC, based on retention time (tR) of a mixture of nC7 to nC40 alkanes (Supelco Analytical).
3. Results and Discussion
3.1. Spectroscopy Characterization
Figure 1 shows the FT-MIR and NMR spectra for the physicochemical characterization of the Melipona capixaba and Melipona quadrifasciata SBH samples. The FT-MIR spectra exhibited absorption frequency from 3500 to 3100 cm–1, which was attributed to O–H stretching from carbohydrates. (24) The presence of carboxylic acid derivatives, including triacylglycerols and fatty acids, in these samples was attributed to infrared bands around 2935 and 1650 cm–1, which are characteristics of the C–H bond with hybridization sp3 and the C═O group. In addition, C–O band was observed in absorption frequency around 1020 cm–1. (25,26) As previously highlighted, the chemical groups detected by the FT-MIR spectroscopy data were related to carbohydrates and lipids. These structures were also detected on 1H NMR spectra analyses from the SBH samples. The signals at δ 1.0 and 1.2 ppm may be attributed to methyl hydrogens and methylene hydrogens from (−CH2−)n hydrocarbon chains of fatty acids and triacylglycerols. The signal at δ 2.1 ppm is characteristic of α-methylene hydrogen linked to carbonyl groups from carboxylic acid, such as acetic acid. In addition, two doublets at δH 4.5 (J = 7.8 Hz) and 5.1 (J = 3.9 Hz) ppm were attributed to β and α hydrogens (H1) in anomeric carbon from d-glucose, respectively. The signals from δH 3.0 to 4.0 ppm were attributed to H2, H3, H4, H5, and H6 from α- and β-d-glucose. (27) Although carbohydrates and lipids, including carboxylic acids, were major compounds found in SBH samples, minor differences were observed in the NMR fingerprinting of M. capixaba and M. quadrifasciata honey.
 ACS Omega 2025, 10, 20, 20550–20561: Figure 1. (a) FT-MIR and (b) NMR spectra of stingless bee honey samples. v─infrared absorption frequencies (stretching or bending).
ACS Omega 2025, 10, 20, 20550–20561: Figure 1. (a) FT-MIR and (b) NMR spectra of stingless bee honey samples. v─infrared absorption frequencies (stretching or bending).
3.2. Volatile Organic Compounds (VOCs)
Figure 3 shows GC–MS TIC, highlighting the most abundant volatile compounds in the SBH samples from M. capixaba and M. quadrifasciata.
 ACS Omega 2025, 10, 20, 20550–20561: Figure 3. GC–MS TIC for selected M. quadrifasciata and M. capixaba stingless bee honey. TPC was expressed in mg GAE 100 g–1 of SBH. (21) 2-Hydroxypropanoic acid ethyl ester. (30) Acetic acid. (44) Hotrienol. (110) 3,5-Dimethoxybenzaldehyde.
ACS Omega 2025, 10, 20, 20550–20561: Figure 3. GC–MS TIC for selected M. quadrifasciata and M. capixaba stingless bee honey. TPC was expressed in mg GAE 100 g–1 of SBH. (21) 2-Hydroxypropanoic acid ethyl ester. (30) Acetic acid. (44) Hotrienol. (110) 3,5-Dimethoxybenzaldehyde.
The GC–MS analyses allowed the identification of VOCs (Supporting Information, Table S1), which were distributed in eight chemical classes: alcohols, aldehydes, carboxylic acids, esters, ethers, phenols, hydrocarbons, and ketones. As observed, carboxylic acids were the most abundant class in all SBH, with values of 74.34 ± 13.03 and 70.95 ± 5.14% for M. capixaba and M. quadrifasciata, respectively, while phenols and hydrocarbons were minor compounds found in these samples (Figure 4).
 ACS Omega 2025, 10, 20, 20550–20561: Figure 4. Box plot for chemical classes of M. quadrifasciata (red bar) and M. capixaba (blue bar) stingless bee honey.
ACS Omega 2025, 10, 20, 20550–20561: Figure 4. Box plot for chemical classes of M. quadrifasciata (red bar) and M. capixaba (blue bar) stingless bee honey.
4. Conclusion
This study conducted a detailed chemical characterization and a comprehensive evaluation of the antioxidant capacity of honey produced by stingless bees of theMelipona genus, collected in the state of Espírito Santo, Brazil. The results revealed a composition rich in carbohydrates, lipids, phenolic compounds, and VOCs.
M. capixaba species exhibit the highest antioxidant capacity. The use of advanced techniques such as FT-MIR, NMR, and GC-MS, combined with spectrophotometric methods FRAP and DPPH, as well as the electrochemical CRAC assay, enabled a robust evaluation of the physicochemical and bioactive properties of SBH.
The data obtained, along with the application of chemometric tools, allowed for clear differentiation of the SBH samples based on their chemical profiles and antioxidant properties. These findings highlight the nutritional and functional value of SBH. Furthermore, this study contributes to the establishment of authenticity and quality standards, essential for regulatory purposes and for promoting the sustainability and global acceptance of SBH.